Abstract

Introduction: Daratumumab is a human, CD38-targeted, IgGκ monoclonal antibody with both direct on-tumor and immunomodulatory mechanisms of action. In the phase 3 POLLUX study, D-Rd reduced the risk of disease progression or death by 63% and significantly increased the overall response rate (ORR) versus Rd alone (93% vs 76%; P <0.001) in RRMM patients (pts). When combined with standard of care regimens across three phase 3 studies including POLLUX, daratumumab demonstrated ≥50% reductions in the risk of progression or death, doubled complete response (CR) rates, and tripled minimal residual disease (MRD)-negative rates at the 10-5 sensitivity threshold in pts with RRMM or newly diagnosed MM (Palumbo A, et al. N Engl J Med 2016. 375[8]:754-766; Dimopoulos MA, et al. N Engl J Med 2016. 375[14]:1319-1331; Mateos MV, et al. N Engl J Med 2018. 378[6]:518-528). Improvements in deeper responses gained from novel MM treatments have led to interest in sustained MRD negativity as a potential surrogate endpoint (Kumar S, et al. Lancet Oncol 2016. 17[8]:e328-e346; Anderson KC. Blood Adv 2017. 1[8]:517-521). Here, we present updated efficacy and safety analyses of POLLUX, including sustained MRD negativity, after >3 years of median follow-up.
Methods: Pts with ≥1 prior line of therapy were randomized (1:1) to receive Rd (lenalidomide 25 mg PO on Days 1-21 of each 28-day cycle; dexamethasone 40 mg per week) ± daratumumab (16 mg/kg IV QW for Cycles 1-2, Q2W for Cycles 3-6, then Q4W until disease progression). High risk cytogenetic abnormalities included t(4;14), t(14;16), and del17p. At the time of suspected CR and at 3 and 6 months after confirmed CR (and every 12 months thereafter if CR was maintained), bone marrow aspirates were assessed for MRD using clonoSEQ® V2.0 (Adaptive Biotechnologies, Seattle, WA). Sustained MRD negativity was defined as maintenance of MRD negativity at the 10-5 threshold for ≥6 or ≥12 months. PFS on subsequent line of therapy (PFS2), an exploratory endpoint, was defined as time from randomization to progression after next line of subsequent therapy or death.
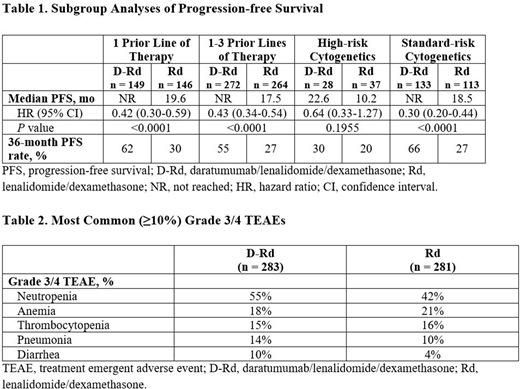
Results: A total of 569 pts were randomized (D-Rd, n = 286; Rd, n = 283). At a median follow-up of 39.5 months, D-Rd significantly prolonged progression-free survival (PFS) versus Rd (median, not reached [NR] vs 17.5 months; hazard ratio [HR], 0.44; 95% confidence interval [CI], 0.35-0.55; P <0.0001); 36-month PFS rate was 55% versus 28%. D-Rd also prolonged PFS versus Rd among pts with 1 prior line of therapy and pts with 1-3 prior lines of therapy (Table 1). A PFS benefit for D-Rd versus Rd was also observed regardless of cytogenetic risk status (Table 1). D-Rd was associated with a significantly higher ORR versus Rd (93% vs 76%), including higher rates of ≥very good partial response (80% vs 49%) and ≥CR (56% vs 23%; all P <0.0001). At the 10-5 sensitivity threshold, MRD-negativity was achieved by 87 (30%) D-Rd pts versus 15 (5%) Rd pts (P <0.000001). Among the ITT population, sustained MRD negativity was achieved by 47 (16%) D-Rd pts versus 2 (0.7%) Rd pts for ≥6 months and 37 (13%) D-Rd pts versus 1 (0.4%) Rd pt for ≥12 months (both P <0.0001). Median time to next therapy for D-Rd versus Rd was NR in the D-Rd group versus 22.8 months in the Rd group (HR, 0.38; 95% CI, 0.29-0.49; P <0.0001). In the D-Rd group, 98 (34%) overall survival (OS) events were observed versus 118 (42%) OS events in the Rd group; OS follow-up is ongoing.
The median duration of treatment was 34.3 months in the D-Rd arm versus 16.0 months in the Rd arm. The most common (≥10%) grade 3/4 treatment-emergent adverse events (TEAEs) observed with D-Rd versus Rd are described in Table 2. No differences were observed for D-Rd versus Rd in discontinuations due to TEAEs (14% of pts in each treatment group) or incidences of second primary malignancies (7% of pts in each treatment group).
Updated data including PFS2 will be presented at the meeting.
Conclusion: After >3 years of median follow-up, D-Rd continued to demonstrate a significant PFS benefit and higher rates of deeper responses versus Rd alone in RRMM pts. The higher rate of sustained MRD negativity with D-Rd compared with Rd suggests that continued D-Rd treatment drives these deep responses and delays progression. No new safety signals were observed following a median of 34 months of D-Rd exposure.
Bahlis:Janssen: Consultancy, Honoraria, Research Funding; Celgene: Consultancy, Honoraria, Research Funding; Amgen: Consultancy, Honoraria, Research Funding. Dimopoulos:Janssen: Honoraria; Bristol-Myers Squibb: Honoraria; Celgene: Honoraria; Amgen: Honoraria; Takeda: Honoraria. White:Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees. Cook:Amgen, Bristol-Myers Squibb, GlycoMimetics, Celgene, Janssen and Takeda and Sanofi: Honoraria; Celgene, Janssen and Takeda: Research Funding. Ho:Takeda: Honoraria, Other: Travel to meeting; Novartis: Honoraria; Amgen: Honoraria; Janssen: Honoraria; Celgene: Other: Travel to meeting. Moreau:Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees; Abbvie: Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees. Krevvata:Janssen Research & Development, LLC: Employment. Chiu:Janssen Research & Development, LLC: Employment. Qin:Janssen Research & Development, LLC: Employment. Okonkwo:Janssen Research & Development, LLC: Employment. Trivedi:Janssen Research & Development, LLC: Employment. Qi:Janssen Research & Development, LLC: Employment. San-Miguel:Janssen: Honoraria; Celgene: Honoraria; Amgen: Honoraria; BMS: Honoraria; Novartis: Honoraria; Sanofi: Honoraria; Roche: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal